Dual efficacy: Rezdiffra delivers statistically significant fibrosis improvement* and steatohepatitis resolution† at Week 521
There were no differences in response to Rezdiffra for both primary endpoints based on age, gender, type 2 diabetes status, GLP‑1 therapy, or fibrosis stage (F2 or F3)1,2
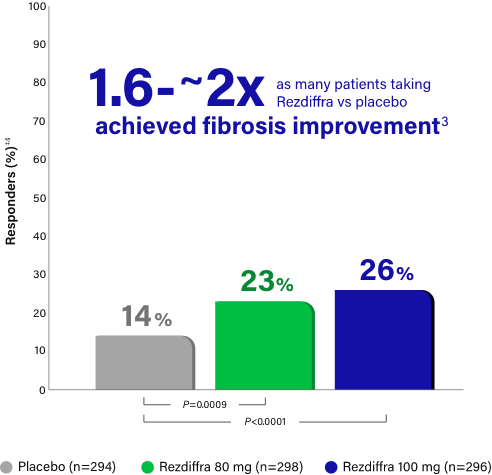
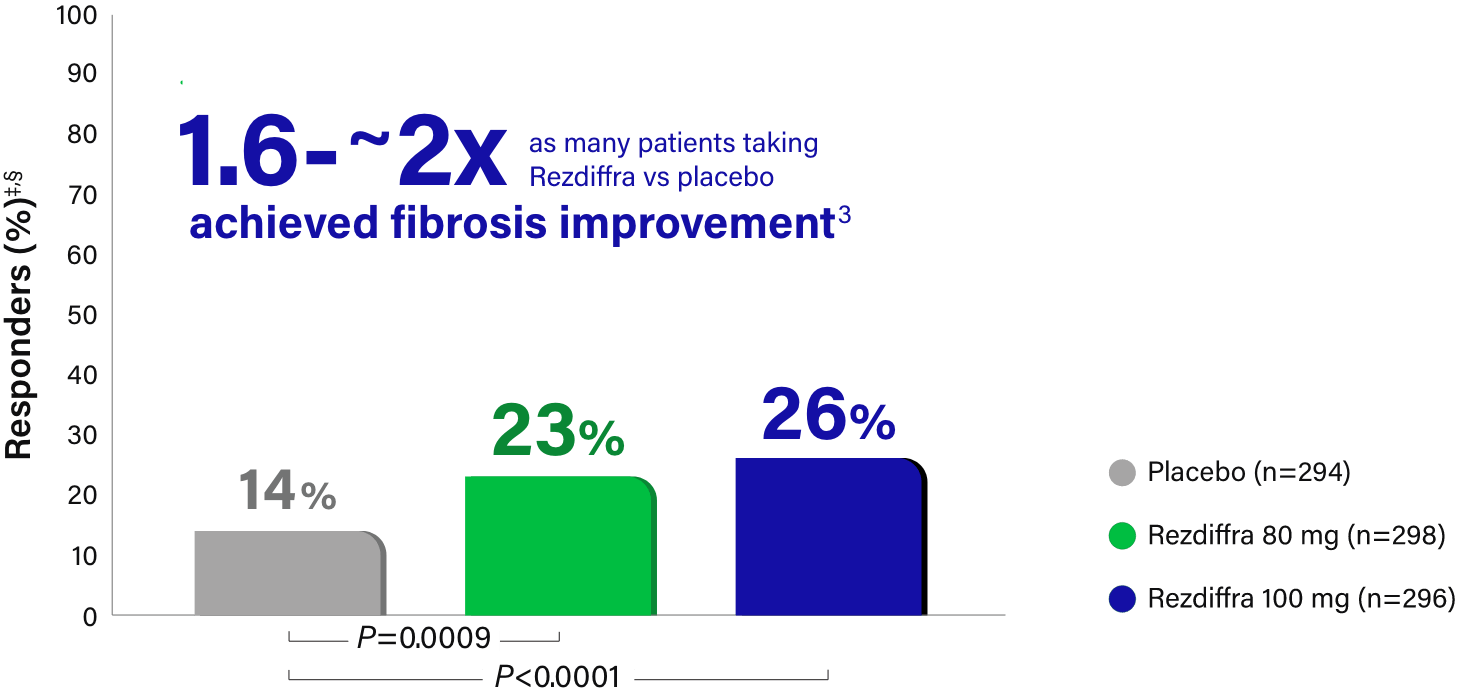
Fibrosis improvement with no worsening of steatohepatitis
| Placebo (n=294) |
Rezdiffra 80 mg (n=298) |
Rezdiffra 100 mg (n=296) |
|
|---|---|---|---|
|
Response rate, Pathologist A (%) |
15 | 23 | 28 |
| Difference in response rate vs placebo (95% CI) | 8 (2, 14) | 13 (7, 20) | |
|
Response rate, Pathologist B (%) |
13 | 23 | 24 |
| Difference in response rate vs placebo (95% CI) | 11 (5, 17) | 11 (5, 17) |
Rezdiffra achieved statistically significant results for fibrosis improvement for both doses in a statistical analysis incorporating both pathologists' independent readings.1,‡
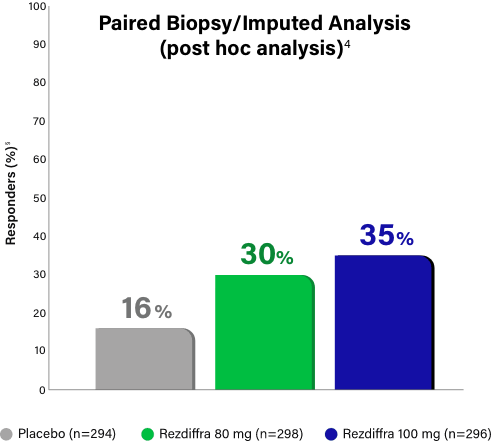
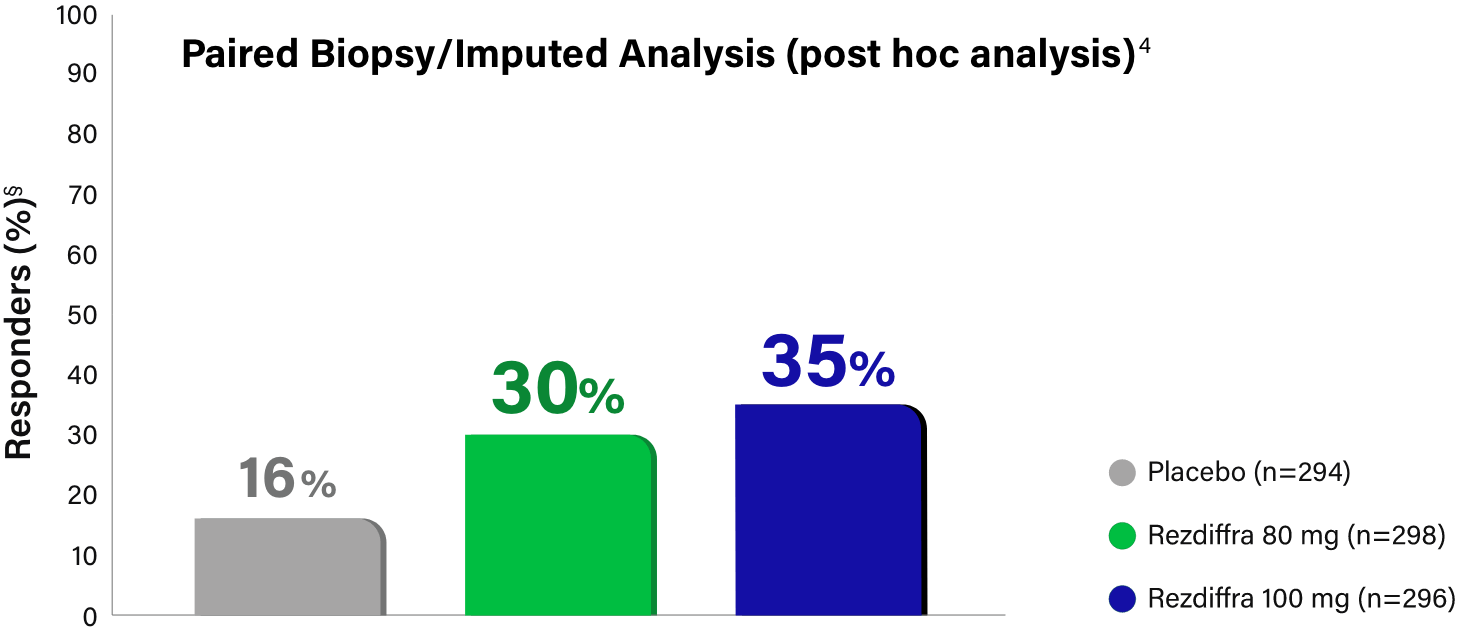
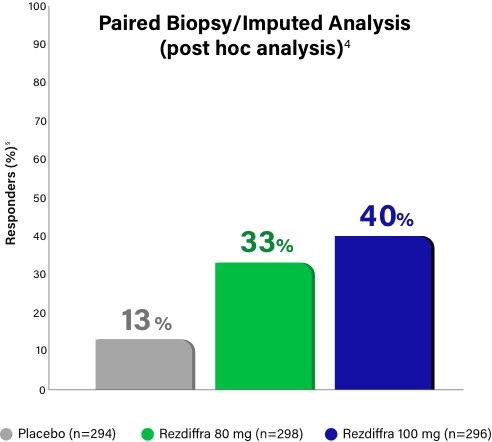
Paired Biopsy/Imputed Analysis Details
In MAESTRO-NASH, patients without a Week 52 liver biopsy were considered an “endpoint non-responder” in the primary endpoint analysis for the intent-to-treat population (N=888).1 Given these “endpoint non-responders” were each randomized to a treatment group and could have potentially responded, the FDA conducted an independent sensitivity analysis, imputing all missing values with a logistic regression model, which adjusted for treatment group, baseline type 2 diabetes (presence or absence), baseline fibrosis stage (F2 or F3), and pathologist.4 Based on Madrigal data, 695 (78.3% of the 888) patients had paired biopsy data at both baseline and Week 52 (n=246, placebo; n=234, 80 mg; n=215, 100 mg), resulting in 193 (21.7%) patients without paired biopsy data (n=48, placebo; n=64, 80 mg; n=81, 100 mg).3
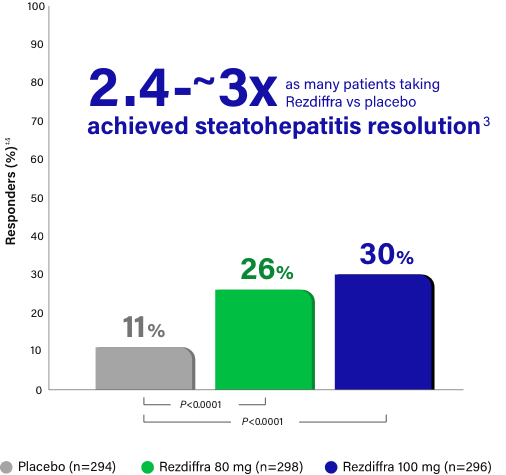
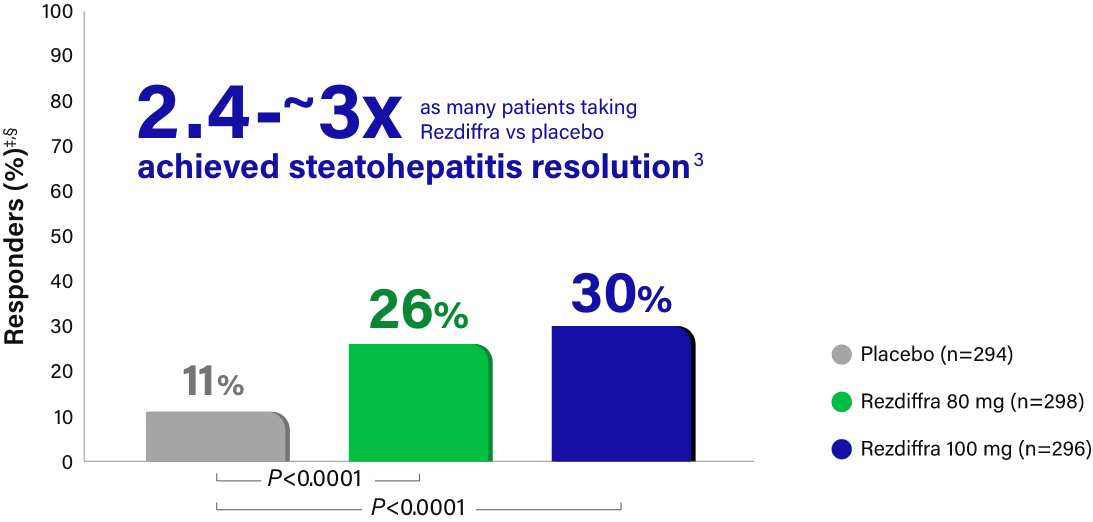
Steatohepatitis resolution with no worsening of fibrosis
| Placebo (n=294) |
Rezdiffra 80 mg (n=298) |
Rezdiffra 100 mg (n=296) |
|
|---|---|---|---|
|
Response rate, Pathologist A (%) |
13 | 27 | 36 |
| Difference in response rate vs placebo (95% CI) | 14 (8, 20) | 23 (16, 30) | |
|
Response rate, Pathologist B (%) |
9 | 26 | 24 |
| Difference in response rate vs placebo (95% CI) | 17 (11, 23) | 15 (9, 21) |
Rezdiffra achieved statistically significant results for steatohepatitis resolution for both doses in a statistical analysis incorporating both pathologists’ independent readings.1,‡
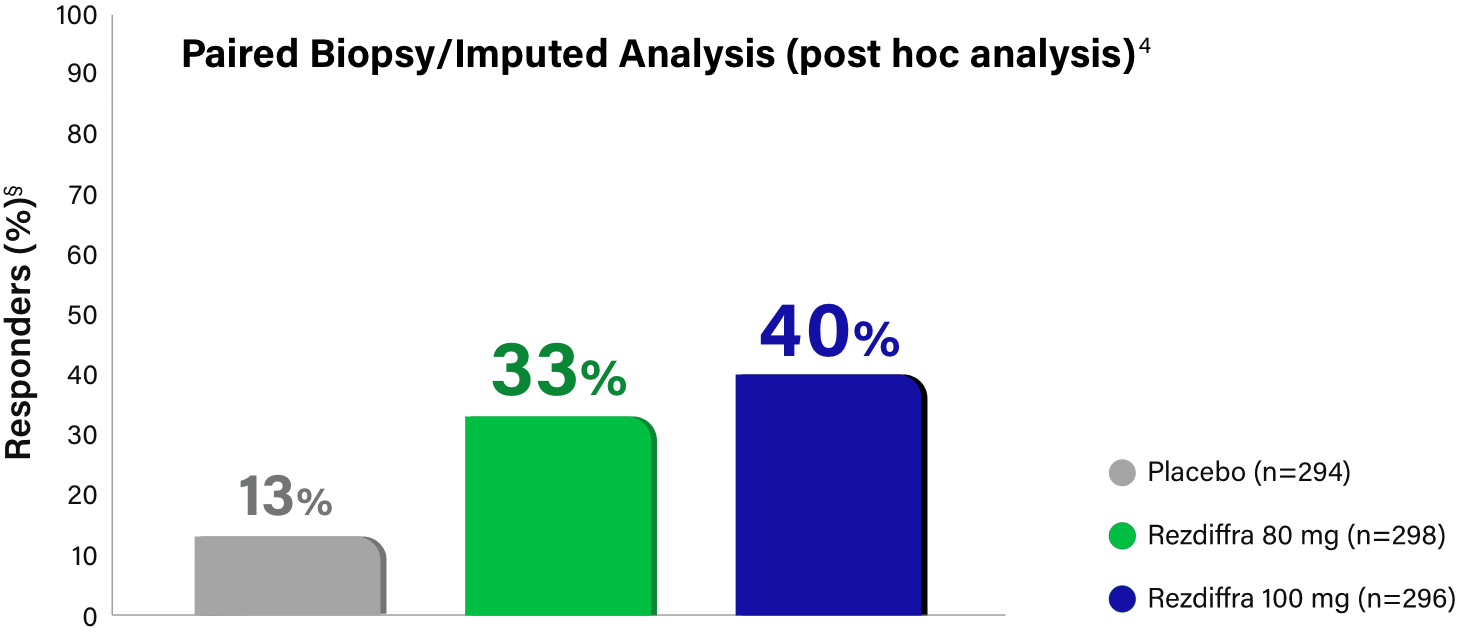
Paired Biopsy/Imputed Analysis Details
In MAESTRO-NASH, patients without a Week 52 liver biopsy were considered an “endpoint non-responder” in the primary endpoint analysis for the intent-to-treat population (N=888).1 Given these “endpoint non-responders” were each randomized to a treatment group and could have potentially responded, the FDA conducted an independent sensitivity analysis, imputing all missing values with a logistic regression model, which adjusted for treatment group, baseline type 2 diabetes (presence or absence), baseline fibrosis stage (F2 or F3), and pathologist.4 Based on Madrigal data, 695 (78.3% of the 888) patients had paired biopsy data at both baseline and Week 52 (n=246, placebo; n=234, 80 mg; n=215, 100 mg), resulting in 193 (21.7%) patients without paired biopsy data (n=48, placebo; n=64, 80 mg; n=81, 100 mg).3
Learn more about the MAESTRO-NASH trial design.
Learn how to identify appropriate patients for Rezdiffra
See CharacteristicsLearn about the demonstrated safety and tolerability profile of Rezdiffra1
See Safety InformationReferences:
- Rezdiffra. Prescribing Information. Madrigal Pharmaceuticals, Inc.
- Harrison SA et al. N Engl J Med. 2024;390(6)(article and suppl):497-509.
- Data on file. REF-00630. Madrigal Pharmaceuticals, Inc.; June 2024.
- Data on file. REF-00923. Madrigal Pharmaceuticals, Inc.; November 2024.