WARNINGS AND PRECAUTIONS
Hepatotoxicity
Hepatotoxicity has been observed with the use of Rezdiffra. One patient developed substantial elevations of
liver biochemistries that resolved when treatment was interrupted. Please see full Prescribing
Information for more details on this specific case of Hepatotoxicity [see Warnings and Precautions
(5.1)].
Monitor for elevations in liver tests, liver-related adverse reactions, and symptoms/signs of hepatotoxicity
(eg, fatigue, nausea, vomiting, right upper quadrant pain or tenderness, jaundice, fever, rash, and/or
eosinophilia [>5%]). If hepatotoxicity is suspected, discontinue Rezdiffra and monitor. If laboratory values
return to baseline, weigh the potential risks against the benefits of restarting Rezdiffra. If laboratory
values do not return to baseline, consider drug-induced autoimmune-like hepatitis (DI-ALH) or autoimmune
liver disease in the evaluation of elevations in liver tests.
Gallbladder-Related Adverse Reactions
Cholelithiasis, acute cholecystitis, and obstructive pancreatitis (gallstone) were observed more often in
Rezdiffra-treated patients than in placebo-treated patients. The exposure-adjusted incidence rates (EAIRs)
for these events were less than 1 per 100 person-years (PY) for all treatment arms. If cholelithiasis is
suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated. If an acute
gallbladder event is suspected, interrupt treatment until the event is resolved.
Drug Interaction with Certain Statins
An increase in exposure of atorvastatin, pravastatin, rosuvastatin and simvastatin was observed when
concomitantly administered with Rezdiffra, which may increase the risk of adverse reactions related to these
drugs.
Dosage adjustment for certain statins is recommended. Monitor for statin-related adverse reactions
including, but not limited to, elevation of liver tests, myopathy, and rhabdomyolysis. Please see the
upcoming Drug Interactions section of the Important Safety Information for more details.
ADVERSE REACTIONS
The most common adverse reactions with Rezdiffra (reported in ≥ 5% of patients and higher compared to
placebo) are diarrhea, nausea, pruritus, vomiting, constipation, abdominal pain, and dizziness. Diarrhea and
nausea were the most common causes of treatment discontinuation.
DRUG INTERACTIONS
Clinically Significant Interactions Affecting Rezdiffra
-
Concomitant use with strong CYP2C8 inhibitors (eg, gemfibrozil) is not recommended. Reduce dosage if
used concomitantly with a moderate CYP2C8 inhibitor (eg, clopidogrel).
-
Concomitant use with OATP1B1 or OATP1B3 inhibitors (eg, cyclosporine) is not recommended.
Clinically Significant Interactions Affecting Other Drugs
-
Statins: Limit daily rosuvastatin and simvastatin dosage to 20 mg. Limit pravastatin
and atorvastatin dosage to 40 mg.
-
CYP2C8 Substrates: Monitor patients more frequently for substrate-related adverse
reactions if Rezdiffra is co-administered with CYP2C8 substrates where minimal concentration changes may
lead to serious adverse reactions.
USE IN SPECIFIC POPULATIONS
Pregnancy
There are no available data on Rezdiffra use in pregnant women. Report pregnancies to Madrigal
Pharmaceuticals, Inc.’s Adverse Event reporting line at 1-800-905-0324 and
https://www.madrigalpharma.com/contact/.
Lactation
There is no information regarding the presence of Rezdiffra in human or animal milk, the effects on the
breast-fed infant, or the effects on milk production. The developmental and health benefits of breastfeeding
should be considered along with the mother’s clinical need for Rezdiffra and any potential adverse effects
on the breastfed infant from Rezdiffra or from the underlying maternal condition.
Geriatric Use
Numerically higher incidence of adverse reactions have been observed in patients ≥65 years of age compared
to younger adult patients.
Renal Impairment
Rezdiffra has not been studied in patients with severe renal impairment.
Hepatic Impairment
Avoid use in patients with decompensated cirrhosis (consistent with moderate to severe hepatic impairment).
Moderate or severe hepatic impairment (Child-Pugh Class B or C) may increase the risk of adverse reactions.
The safety and effectiveness have not been established in patients with cirrhosis.
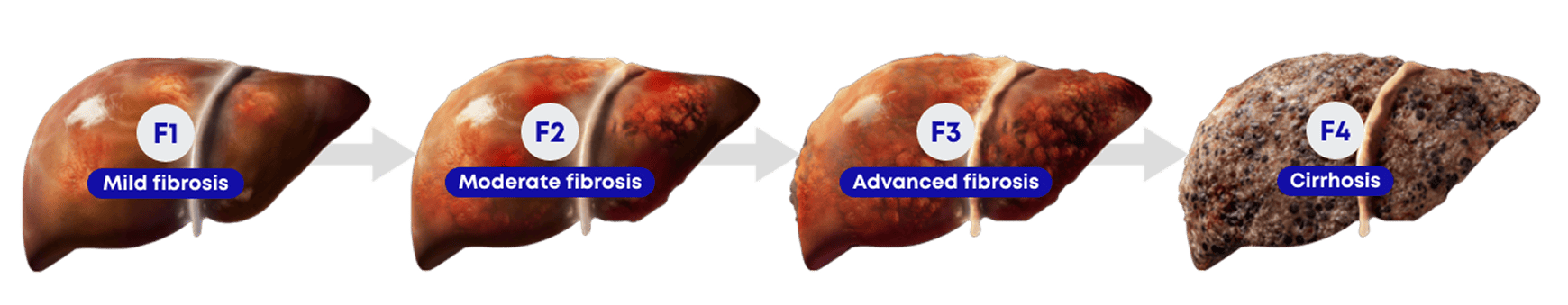
INDICATION
Rezdiffra is indicated in conjunction with diet and exercise for the treatment of adults with noncirrhotic
metabolic dysfunction-associated steatohepatitis (MASH) with moderate to advanced liver fibrosis (consistent
with stages F2 to F3 fibrosis).
This indication is approved under accelerated approval based on improvement of MASH and fibrosis. Continued
approval for this indication may be contingent upon verification and description of clinical benefit in
confirmatory trials.
Limitation of Use: Avoid use in patients with decompensated cirrhosis.
Please see full Prescribing Information for Rezdiffra.